Indian Meds RX Review: Trusted Pharmacy Ratings & Experiences
Dec 26 2023 - Health Product Reviews
When you hear generic phenytoin, a generic version of the antiseizure drug phenytoin used to control epilepsy and prevent seizures. Also known as phenytoin sodium, it's one of the most widely prescribed generic medications for long-term seizure control. But here’s the thing: just because it’s generic doesn’t mean it’s interchangeable without consequences. Many people assume all versions of phenytoin work the same way, but small differences in how the body absorbs the drug can lead to breakthrough seizures or toxic side effects. This isn’t theoretical—it’s backed by real patient reports and FDA alerts.
The path to getting generic phenytoin, an FDA-approved version of the brand-name drug Dilantin. Also known as phenytoin sodium, it's one of the most widely prescribed generic medications for long-term seizure control. approved isn’t simple. It goes through an ANDA, Abbreviated New Drug Application, the FDA’s official pathway for approving generic medications without repeating costly clinical trials. Also known as Abbreviated New Drug Application, it’s the standard process for bringing low-cost versions of brand-name drugs to market.. Manufacturers must prove their version is therapeutically equivalent to the original—meaning it delivers the same amount of active ingredient at the same rate. But bioequivalence doesn’t always mean clinical equivalence. Some patients report seizures returning or side effects worsening after switching brands, even when both are labeled as generic phenytoin. That’s why pharmacists and doctors need to track which specific generic version a patient is using, and why insurance companies pushing for automatic substitution can be risky.
It’s not just about phenytoin. The same issues show up with other narrow-therapeutic-index drugs like warfarin, levothyroxine, and cyclosporine. The FDA Orange Book, the official government database that lists approved drug products with therapeutic equivalence evaluations. Also known as Therapeutic Equivalence Evaluations, it’s the tool pharmacists use to determine which generics can be substituted without a doctor’s approval. tells you which versions are rated as AB-equivalent, but it doesn’t tell you how a specific patient will react. That’s why continuing education for pharmacists focuses so heavily on therapeutic equivalence and substitution rules. Patients need to know: if your doctor prescribed generic phenytoin, don’t switch brands without checking with your pharmacy or provider. One batch might work perfectly, the next might not. And if you’re on multiple meds, interactions with other drugs—like antibiotics or antifungals—can change how phenytoin behaves in your body.
What you’ll find in this collection are real, practical guides on how generic drugs work behind the scenes, how to spot when a substitution might be dangerous, and what to ask your pharmacist before accepting a new bottle. From how the Hatch-Waxman Act shaped today’s generic market, to why some patients end up with higher blood levels after a switch, to how insurers pressure pharmacies to change meds without warning—this isn’t just theory. It’s what’s happening in clinics, pharmacies, and homes every day. You’re not just reading about drugs. You’re learning how to protect your health when the system pushes for cost savings over consistency.
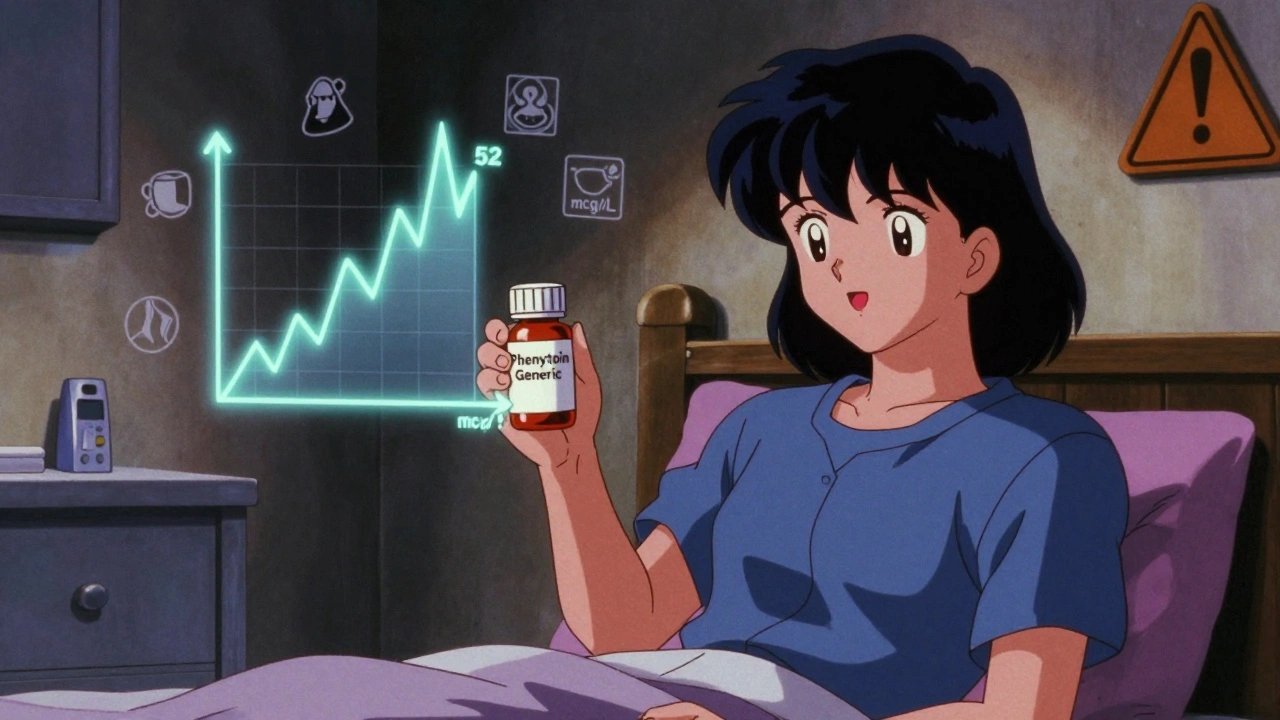
Switching between brand and generic phenytoin can cause dangerous shifts in blood levels due to its narrow therapeutic window and nonlinear metabolism. Therapeutic drug monitoring is essential after any formulation change to prevent toxicity or seizure breakthrough.
read more© 2026. All rights reserved.
