When a drug goes directly into your bloodstream, there’s no second chance. Unlike pills that pass through the stomach and liver, injectables bypass every natural defense. That’s why sterile manufacturing for injectables isn’t just about cleanliness-it’s about survival. A single microbe in a vial can trigger sepsis, organ failure, or death. The 2012 meningitis outbreak linked to contaminated steroid injections killed 64 people and sickened over 750. That tragedy didn’t come from negligence alone-it came from systems that weren’t built to handle the extreme demands of sterile production.
Why Sterile Manufacturing Is Different
Oral medications can tolerate some level of contamination. Your body has filters-stomach acid, immune cells, liver enzymes. Injectables don’t. They enter the blood directly. So the standard isn’t "clean enough" or "mostly sterile." It’s sterile-with a probability of contamination less than one in a million (SAL 10^-6). That’s not a suggestion. It’s a requirement from the WHO, FDA, and EU regulators. This isn’t new. After the 1955 Cutter Laboratories polio vaccine disaster, where live virus slipped through, the FDA created the first formal GMP rules. Today, those rules are stricter than ever. The EU’s revised Annex 1 (2022) and FDA’s 2023 guidance demand continuous environmental monitoring, real-time data, and risk-based controls. You can’t just test at the end anymore. You have to prove sterility is built into every step.Two Paths to Sterility: Terminal vs. Aseptic
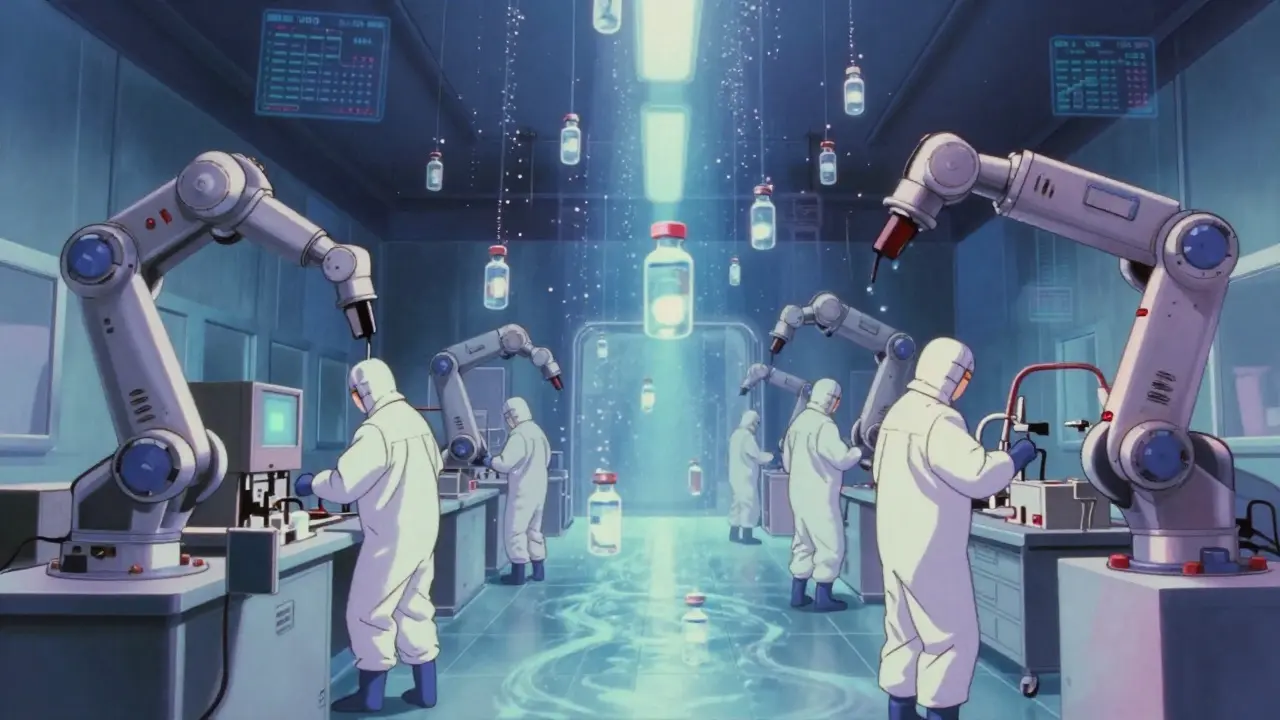
There are two main ways to make sterile injectables: terminal sterilization and aseptic processing. They’re not interchangeable. Each has trade-offs. Terminal sterilization means you make the product, seal it, then kill everything inside with heat or radiation. Steam at 121°C for 15-20 minutes is the gold standard. It gives you a sterility assurance level of 10^-12-far better than the required 10^-6. It’s reliable, repeatable, and cheaper. But here’s the catch: only 30-40% of injectables can survive it. Biologics like monoclonal antibodies, vaccines, and gene therapies? They’ll denature. They’ll fall apart. Heat destroys them. That’s where aseptic processing comes in. No heat. No radiation. Instead, everything-the drug, the vials, the stoppers, the air-is kept sterile from start to finish. This happens in ISO 5 cleanrooms (Class 100), where particle counts are capped at 3,520 per cubic meter for particles 0.5μm and larger. Air flows in one direction, like a silent waterfall, sweeping contaminants away. Operators wear full-body suits, move slowly, and never turn their backs on the product. Aseptic processing is more expensive-up to $150,000 per batch versus $50,000 for terminal. But it’s the only way to make modern drugs. Over 40% of new drug approvals in 2023 required sterile injectable formats. And nearly a third of those were monoclonal antibodies. You can’t sterilize them after filling. You have to fill them sterile.What the Cleanroom Demands
An ISO 5 cleanroom isn’t just a fancy room with filters. It’s a precision machine. Every parameter matters:- Temperature: 20-24°C. Too cold, and condensation forms. Too warm, and people sweat more.
- Humidity: 45-55%. Dry air causes static; humid air invites mold.
- Pressure: 10-15 Pascals higher than adjacent rooms. Air flows inward, never outward.
- Air changes: 20-60 per hour. That’s 100% of the room’s air replaced every 1-3 minutes.
- Personnel: No more than 1-2 people in the ISO 5 zone during filling. Every movement creates particles.

Testing for Sterility: Media Fills and Monitoring
You can’t just look at a vial and know it’s sterile. You have to simulate the process. That’s called a media fill. You replace the drug with nutrient broth, run the entire filling process, then incubate the vials for 14 days. If anything grows, you failed. FDA requires media fills to include 5,000-10,000 units per run. That’s not a sample. That’s a full-scale rehearsal. If you get even one contaminated vial in a batch of 10,000, your process is broken. The FDA says failure rates above 0.1% mean your aseptic technique is inadequate. Air monitoring is continuous now. Sensors count particles every second. Air samplers catch microbes in real time. Alert levels are set at 1 CFU/m³. Action levels at 5 CFU/m³. Go above that, and production stops. No exceptions.Costs, Failures, and Real-World Pain Points
Sterile manufacturing isn’t cheap. Setting up a small facility costs $50-100 million. Training staff takes 40-80 hours per person, plus semi-annual media fill qualifications. Documentation runs 250-300 pages per batch. 15-20% of that is dedicated to sterility records. Failures are devastating. One sterility test failure averages $1.2 million in losses. In 2023, a top pharma company lost $450,000 in one batch because a glove in their RABS system had a microscopic tear. Another company spent $2.5 million on automated visual inspection to drop defect rates from 0.2% to 0.05%. The FDA’s 2022 inspection data shows 68% of sterile manufacturing violations are tied to aseptic technique. Only 12% involve terminal sterilization. That tells you where the real risk lies.
Technology Is Changing the Game
The industry is moving fast. Isolators-sealed, robotic systems where operators work through gloves-are replacing traditional cleanrooms. They cut contamination risk by 100 to 1,000 times, according to experts. But they cost 40% more to install. Closed processing systems are now in 65% of new facilities. No more opening vials by hand. No more exposed transfers. Everything happens in sealed tubes. That’s a huge win. Rapid microbiological methods are cutting test times from 14 days to 24 hours. Digital twins simulate every step before you run a batch. AI tools are being tested to predict contamination risks before they happen. The EU and FDA are pushing for continuous monitoring, real-time data, and automated decision-making. By 2025, every sterile facility will need $15-25 million in upgrades to meet Annex 1. If you’re not investing, you’re falling behind.Who Makes These Drugs Today?
Most sterile injectables aren’t made by big pharma anymore. Contract manufacturers-CDMOs like Catalent, Lonza, and Thermo Fisher-handle 55% of production. That’s because building and maintaining sterile lines is too expensive and risky for most companies to do alone. But even CDMOs struggle. Only 28 of 1,200 Chinese sterile facilities passed FDA inspections in 2022. India’s capacity is growing, but regulatory alignment lags. Global demand is rising-sterile injectables hit $225 billion in 2023 and are projected to reach $350 billion by 2028. But quality isn’t keeping pace with quantity.What This Means for Patients and Providers
You don’t need to know the difference between RABS and isolators. But you should know that if a drug is injected, it’s held to the highest standard in medicine. The systems behind it are complex, costly, and unforgiving. When you get a shot, you’re trusting a chain of controls that involve hundreds of checks, thousands of data points, and decades of lessons learned from tragedy. The next time you hear about a drug recall, ask: Was it a sterile product? If yes, then the failure wasn’t just a mistake. It was a system breakdown. And the cost wasn’t just financial-it was human.Every sterile injectable you receive was made under conditions most people never see. And that’s exactly how it should be.
What is the difference between terminal sterilization and aseptic processing?
Terminal sterilization kills microbes after the product is sealed, using heat or radiation. It’s reliable and cheaper but only works for products that can withstand high temperatures, like saline solutions. Aseptic processing keeps everything sterile from start to finish without heat, making it the only option for heat-sensitive drugs like biologics and vaccines. Aseptic processing is more complex and expensive but essential for modern injectables.
Why are ISO 5 cleanrooms required for aseptic filling?
ISO 5 cleanrooms limit airborne particles to 3,520 per cubic meter at 0.5μm or larger. This level of control is necessary because even tiny particles can carry microbes. In aseptic filling, operators are the biggest source of contamination. ISO 5 environments, with unidirectional airflow and strict access controls, reduce that risk to a level where sterility can be reliably maintained without terminal sterilization.
What happens if a sterile injectable fails a sterility test?
The entire batch is rejected and destroyed. No exceptions. Even one positive result means the product may contain live microorganisms. Regulatory agencies require full investigation into the root cause-was it a glove tear? A failed air filter? Improper gowning? The findings must be documented, corrected, and validated before future batches can be released. This is why media fill failures cost companies hundreds of thousands of dollars.
How often are sterile manufacturing facilities inspected?
Regulators like the FDA inspect sterile facilities every 2-3 years on average, but inspections can happen more frequently if there are prior issues or complaints. In 2022, FDA citations for sterile manufacturing rose to 1,872 from 1,245 in 2019, showing increased scrutiny. Facilities with recent deviations or product recalls are often prioritized for unannounced inspections.
Can sterile injectables be made outside of the U.S. or EU?
Yes, but they must meet the same standards. Facilities in countries like India, China, and Brazil can manufacture sterile injectables for global markets, but they must pass FDA or EMA inspections. Only 28 of 1,200 Chinese sterile facilities passed FDA inspections in 2022. Many fail due to inadequate environmental controls, poor documentation, or lack of real-time monitoring. Compliance isn’t optional-it’s a global requirement.
For facilities planning upgrades, the key is to invest in automation, continuous monitoring, and staff training-not just equipment. The biggest risk isn’t outdated machinery. It’s human error. And that’s something no machine can fully fix.



Dana Termini - 6 January 2026
Just thinking about how much goes into a single injection gives me chills. Every vial is a fortress of science, and we barely notice. The fact that we can get life-saving drugs without thinking about the 250-page documentation behind them is insane.
Pavan Vora - 6 January 2026
India has so many facilities, but so few pass FDA… it’s heartbreaking. We have the manpower, the will, but the infrastructure? Not always. I’ve seen labs where the air filters are old, and they still say ‘ISO 5’… please, someone audit them properly. It’s not just money-it’s lives.
Stuart Shield - 8 January 2026
It’s like watching a ballet where every step could kill you. The way air flows like a silent river, operators moving like ghosts in full-body suits, vials baked at 250°C… it’s not manufacturing. It’s alchemy with a death penalty if you blink wrong. And yet, we treat it like it’s just another drug. We owe these people more than applause-we owe them respect.
Indra Triawan - 9 January 2026
Isn’t it ironic? We pour billions into making something sterile, yet we live in a world that glorifies ‘natural’ remedies that are literally crawling with microbes. We fear GMOs but trust a herbal tincture from a guy who washes his hands in rainwater. The cognitive dissonance is beautiful. Or tragic. I can’t tell anymore.
Joann Absi - 11 January 2026
USA built this. FDA didn’t just write rules-they built a wall between life and death. And now? We outsource to countries that can’t even spell ‘sterility’ right. China’s 28 out of 1,200? That’s not a failure. That’s a betrayal. If you can’t do it right, don’t do it at all. We’re not selling coffee here.
Mukesh Pareek - 12 January 2026
The SAL 10^-6 isn't a target-it's a baseline. Aseptic processing requires a risk-based approach with real-time environmental monitoring (RTEM), PAT tools, and closed-system integration. Without dynamic containment and microbial burden control, you're not compliant-you're negligent. The 2022 Annex 1 mandates process analytical technology (PAT) for critical process parameters (CPPs). If you're still relying on end-product testing, you're in the 1980s.
Ashley S - 12 January 2026
So we spend millions so a shot doesn’t kill us? Wow. Can we just use pills like normal people? This is ridiculous. Someone’s making bank off fear.
Jeane Hendrix - 14 January 2026
Media fills are wild-14 days of waiting just to see if something grew. And the fact that one torn glove can wipe out a $450k batch? It’s terrifying. But I think the real win is AI predicting contamination before it happens. We’re not just reacting anymore-we’re anticipating. That’s the future. And honestly? I’m kinda proud of that.
Rachel Wermager - 15 January 2026
Terminal sterilization at 121°C gives SAL 10^-12? That’s overkill. But it’s the only method that doesn’t rely on human behavior. Aseptic processing? It’s a 70% failure rate waiting to happen. Operators are the largest contamination source-no amount of training fixes biology. Isolators aren’t a luxury-they’re the only ethical path forward. If your facility still uses open RABS, you’re gambling with patient lives. And that’s not GMP. That’s gambling.