By 2026, more than 40% of chronic disease management plans will include digital therapeutics alongside traditional medications. That’s not a prediction-it’s happening right now. Patients with diabetes, anxiety, asthma, and opioid use disorder are using apps and software as part of their treatment, not just as reminders, but as active medical tools. And here’s the problem: we still don’t know enough about how these digital tools interact with the pills they’re supposed to help you take.
What Exactly Are Digital Therapeutics?
Digital therapeutics (DTx) aren’t fitness trackers or meditation apps. They’re FDA-cleared medical treatments delivered through software. Think of them as prescription apps-approved by regulators, tested in clinical trials, and designed to change health outcomes. The first one, reSET for substance use disorder, got clearance in 2018. Since then, over 50 have been approved, including DaylightRx for generalized anxiety disorder, cleared in September 2024.
These aren’t just reminders to take your pills. They use cognitive behavioral therapy (CBT), real-time feedback loops, AI-driven personalization, and even virtual reality to treat conditions. For example, DarioEngage helps people with diabetes adjust insulin doses based on blood sugar trends. EndeavorRx is a video game approved for pediatric ADHD that improves attention through neurostimulation. These aren’t gimmicks-they’re clinical interventions.
How DTx Improve Medication Adherence
One in three prescriptions are never filled. Even when filled, half of patients with chronic conditions miss doses regularly. That’s why DTx are gaining traction. They don’t just nudge you-they track, analyze, and respond.
Apps like Medisafe monitor whether you’ve picked up your meds, scanned your pill bottle, or logged a dose. When you skip a dose, the app doesn’t just send a notification. It asks why. Is it cost? Fear of side effects? Confusion about timing? Then it connects you to financial aid, explains side effects, or adjusts reminders based on your schedule. Studies show these apps boost adherence by up to 25% in conditions like diabetes and mental health disorders.
Compare that to traditional methods. Pharmacies calling patients to check if they filled their prescription? Only 15-20% improvement. DTx outperforms this by a wide margin. For asthma and COPD, where adherence is often below 50%, DTx pushes it up to 72-78%. That’s not a small win-it’s life-changing.
When DTx and Medications Collide
Here’s where things get tricky. DTx aren’t passive tools. They actively influence behavior, emotions, and physiology. And that can interact with medications in ways we’re only beginning to understand.
Take DaylightRx, the FDA-approved CBT app for anxiety. It works by rewiring thought patterns. But what if you’re also taking an SSRI like sertraline? Could the app’s intense exposure exercises increase anxiety temporarily, making side effects from the medication feel worse? Or worse-could the app’s calming techniques reduce your need for the medication, leading you to stop taking it too soon?
There’s no official database tracking these interactions. Unlike drugs, which undergo years of testing with hundreds of co-administered medications, DTx are often cleared based on small studies focused on the app alone. The FDA doesn’t yet require combination therapy trials for DTx used alongside drugs. That means doctors are flying blind.
One 2023 study found that 7% of patients using EndeavorRx for ADHD reported headaches, dizziness, or emotional reactions-rates higher than the control group. Were these side effects from the game? Or from interactions with stimulant medications like Adderall? We don’t know.
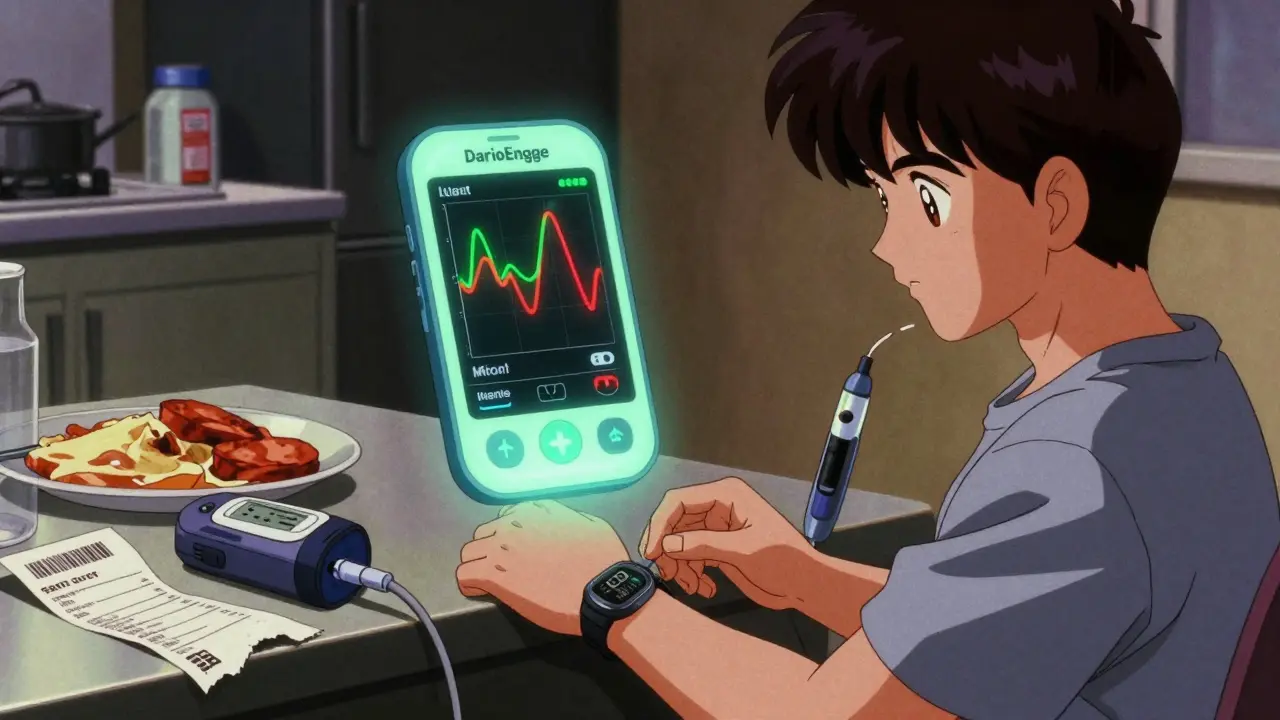
Real-World Patient Stories
Reddit threads from r/diabetes and r/mentalhealth tell a story of hope and frustration.
One user wrote: “DarioEngage helped me drop my HbA1c by 1.8% in six months. My endo adjusted my insulin based on the app’s data. I’ve never felt more in control.”
Another said: “DaylightRx felt robotic. It didn’t address why my anxiety spiked after starting fluoxetine. I stopped using it after two weeks.”
On G2, users praise DTx for real-time support: “When I got a bruise from warfarin, the app asked if I wanted to talk to my doctor. I did. They lowered my dose.” But 62% of negative reviews mention poor integration with pharmacy systems. You can log your meds in the app, but your pharmacy still doesn’t know you’re enrolled. Refills get delayed. Insurance denies coverage. The system doesn’t talk to itself.
Who Struggles With DTx-and Why
DTx works best for people who are digitally literate, motivated, and have stable tech access. That’s not everyone.
A 2024 study in the Journal of Managed Care & Specialty Pharmacy found that 45% of patients over 65 stopped using DTx within a month if they didn’t get in-person help. For those over 70, 38% quit within 30 days without a “DTx navigator”-a staff member trained to walk them through setup, troubleshoot glitches, and link app data to medication decisions.
Even when patients stick with it, providers often don’t know how to interpret the data. A diabetes app might show a pattern of nighttime lows. But without training, a clinician might just see a number and say, “Take less insulin.” They don’t realize the app detected stress-induced cortisol spikes, not poor dosing.

Regulation and the Wild West of Digital Health
The FDA treats DTx as medical devices, not drugs. That means they need 510(k) clearance or De Novo classification-but they don’t need the same level of long-term safety data as a new pill. A drug might be tested on 10,000 people over five years. A DTx might be cleared based on a 200-person study over six months.
And then there’s the gray area. Thousands of apps claim to “help with anxiety” or “manage diabetes.” Most aren’t regulated. They’re sold as wellness tools. But patients don’t know the difference. They download one, assume it’s safe, and combine it with their prescriptions-without telling their doctor.
Regulators are catching up. The FDA plans new guidance in Q2 2025, specifically on how DTx should be studied when used with medications. The European Union’s MDR adds another layer-different rules, different standards. Global fragmentation means a DTx approved in the U.S. might be banned in Germany.
The Future: DTx as Part of the Treatment Formula
The next five years will change everything. By 2027, 65% of specialty pharmacy prescriptions will require a digital companion to qualify for insurance coverage. That’s not speculation-it’s what Medisafe and other major players are already building toward.
Imagine this: Your insulin pump syncs with your DTx app. The app notices your blood sugar drops every time you skip lunch. It alerts your doctor, who adjusts your dose. At the same time, your anxiety app detects elevated heart rate and cortisol levels after a stressful meeting. It suggests a breathing exercise-and tells your psychiatrist you might need a lower dose of your SSRI.
This is precision medicine, powered by software. DTx can collect real-time data on behavior, sleep, mood, and physiology-far more than a blood test or a clinic visit. That data can inform dosing, timing, and even drug selection.
But only if we build the systems to handle it. We need interoperable EHRs. We need training for providers. We need patient support. And we need clinical studies that test DTx + drugs together-not in isolation.
What You Should Do Right Now
If you’re using a DTx app alongside medications:
- Tell your doctor. Don’t assume they know you’re using it.
- Ask if it’s FDA-cleared. If it’s not, it’s not a medical treatment-it’s a wellness tool.
- Track how you feel. Are symptoms improving? Or getting worse after using the app?
- Check for integration. Can your app connect to your pharmacy or EHR? If not, you’re managing two separate systems.
- Request support. If you’re over 65 or not tech-savvy, ask your clinic for a DTx navigator.
If you’re a provider:
- Learn the difference between wellness apps and regulated DTx.
- Start asking patients: “Are you using any apps to manage your condition?”
- Use DTx data as a clinical tool-not a replacement for judgment.
- Advocate for reimbursement and integration in your EHR system.
Digital therapeutics aren’t the future. They’re here. But they’re not magic. They’re medicine-and like all medicine, they come with risks, benefits, and interactions. Ignoring those interactions is dangerous. Understanding them is the next step in real, patient-centered care.
Are digital therapeutics the same as wellness apps?
No. Wellness apps-like meditation or step counters-are not regulated and don’t need clinical proof. Digital therapeutics (DTx) are FDA-cleared medical treatments. They must prove effectiveness in clinical trials and are prescribed like drugs. Look for FDA clearance or a prescription requirement to know it’s a true DTx.
Can DTx replace my medication?
Sometimes, but rarely. DaylightRx, for example, is approved as a standalone treatment for anxiety. But most DTx are designed to work with medication-not replace it. They improve adherence, reduce side effects, or provide behavioral support. Never stop or change your medication without talking to your doctor, even if the app says you’re doing better.
Do DTx interact with my medications?
They can. DTx influence behavior, stress levels, sleep, and even physiology. These changes can affect how your body responds to drugs. For example, an anxiety app that reduces cortisol might lower your need for a beta-blocker. Or a diabetes app that improves sleep might make insulin more effective. But no official database tracks these interactions yet. Always tell your doctor what apps you’re using.
Why do some patients stop using DTx?
The top reasons are poor integration with pharmacies or EHRs, lack of personalization, and no human support. Patients over 65 are especially likely to quit without a navigator. If the app feels generic, doesn’t respond to your needs, or keeps crashing, it’s not working-and you’re not failing. The system is.
Is DTx covered by insurance?
Some are, but not all. Medicare and private insurers are slowly covering FDA-cleared DTx for conditions like diabetes, ADHD, and substance use disorder. Coverage depends on your plan, your diagnosis, and whether your provider prescribes it. Always check with your insurer and ask your doctor to submit the proper codes. Non-prescription apps are rarely covered.



Priya Patel - 10 January 2026
This is actually huge. I’ve been using DaylightRx for my anxiety and my SSRIs feel way more manageable now. Not saying it’s magic, but when the app notices I’m spiraling after a bad night’s sleep and suggests a 5-minute breathing thing? It’s like having a therapist in my pocket. My doc didn’t even know I was using it till I showed him the data.
Vincent Clarizio - 10 January 2026
Let’s be real-this isn’t medicine, it’s tech bro fantasy dressed up in white coats. You think an app that asks you ‘why did you skip your dose?’ is gonna fix systemic healthcare collapse? The FDA clears these things based on 200-person studies while real drugs go through 10,000-patient trials and decades of post-market surveillance. And now we’re supposed to trust that a video game for ADHD doesn’t mess with Adderall? Please. The real problem isn’t the apps-it’s that we’ve outsourced critical thinking to Silicon Valley and now we’re paying for it in unmonitored physiological interactions. This isn’t innovation. It’s negligence with a UI.
Jennifer Littler - 11 January 2026
As a clinical informaticist, I’ve seen this play out in EHRs. DTx data is siloed, unstructured, and often unreadable by providers. We get CSV exports from apps that don’t map to SNOMED codes. So when a patient says ‘my app says my glucose is dropping at night,’ the clinician sees a spreadsheet with no context. No one’s training docs on how to interpret behavioral data streams. We’re building the plane while flying it, and the cockpit’s missing half the gauges.
Alfred Schmidt - 12 January 2026
So let me get this straight-you’re telling me I should trust an app that tells me to take less insulin because it ‘detected stress’? I’ve been on warfarin for 12 years and I’ve seen more bad tech than a hospital cafeteria. These apps crash, lie about data, and then blame the patient when they miss a dose. And now you want me to tell my doctor I’m using one? Like he’s gonna care? He barely looks at my labs. This is a distraction. A shiny object. A distraction from the fact that we still can’t get people to fill prescriptions because they can’t afford them. Stop fetishizing tech. Fix the system.
Sean Feng - 13 January 2026
DTx is just wellness apps with a prescription label. No one’s testing interactions. No one’s tracking outcomes long term. And now insurance wants to force them? Great. More apps. More confusion. More junk.
Priscilla Kraft - 15 January 2026
Just wanted to say thank you for writing this. I’m a 68-year-old with type 2 and DarioEngage literally saved me. My HbA1c dropped from 9.2 to 6.8 in 8 months. But I almost quit after the first week because I couldn’t figure out how to sync it with my phone. My clinic had a DTx navigator who came over and set it up with me-she even called my pharmacy to make sure they knew I was enrolled. If that hadn’t happened, I’d have given up. This tech works-but only if we support people using it. Not just throw it at them and say ‘here, figure it out.’ 🙏
Christian Basel - 16 January 2026
The regulatory framework is a mess. FDA treats DTx as Class II devices, which means they’re subject to 510(k) clearance-basically a ‘substantial equivalence’ claim to something already on the market. But when you combine that with pharmacokinetic variables from SSRIs or anticoagulants, you’re entering pharmacodynamic territory. No one’s mandated interaction trials. That’s not innovation. That’s clinical gambling with patient outcomes.
Alex Smith - 18 January 2026
So let me get this straight-you’re telling me a video game that makes kids pay attention is now a first-line ADHD treatment? And it’s supposed to play nice with Adderall? I’m not saying it doesn’t work-I’m saying it’s terrifying that we’re deploying this without knowing how it interacts with neurochemistry. We’re treating kids like beta testers for a Silicon Valley product. And the worst part? The parents don’t even know the difference between a game and a drug. Welcome to 2026.
Adewumi Gbotemi - 18 January 2026
Here in Nigeria, we don’t even have stable internet for basic telehealth. But I read this and I thought-maybe one day, someone like me can use this. Not because it’s fancy, but because it could help someone who can’t see a doctor every week. I hope someone builds this for places like mine-not just for people with iPhones and insurance.
Michael Patterson - 19 January 2026
Wow this is so deep. I mean, like, really deep. I’ve been using DaylightRx and I think it’s changed my life. But I also think the FDA should require all DTx to have a black box warning like opioids. Because what if the app makes you feel better so you stop your meds? And then you crash? And then you die? And then the company says ‘we didn’t test that interaction’? That’s not innovation, that’s a lawsuit waiting to happen. And also, why do these apps always look like they were designed by a 14-year-old with Canva? Just saying.