When you get a safety communication about a drug, medical device, or public health risk, it’s not enough to just read it and forget about it. These alerts - issued by the FDA, CDC, or WHO - are warning signs. They mean something could be wrong, and your body might be reacting. Ignoring them can lead to serious harm. The real work starts after you read the alert: monitoring your symptoms carefully, consistently, and correctly.
Understand What the Safety Communication Means
Not all safety communications are the same. Some are about rare side effects from a new medication. Others warn about faulty medical devices, contaminated batches, or emerging disease patterns. The key is to know what you’re looking for. For example, if the FDA issues a safety alert about a blood pressure medication linked to liver inflammation, you need to know the specific symptoms: unexplained fatigue, yellowing skin, dark urine, or pain in the upper right abdomen. If it’s about a pacemaker with a battery defect, you might watch for dizziness, irregular heartbeat, or sudden loss of device alerts. The alert should list the exact symptoms to monitor. If it doesn’t, go to the official source - FDA.gov, CDC.gov, or your doctor’s office. Don’t rely on social media or news headlines. Misinformation spreads faster than the risk itself.Choose the Right Monitoring Method
There are two main ways to track symptoms: active and passive. Active monitoring means someone checks in with you regularly - usually daily - via phone, text, or an app. This is common for healthcare workers after exposure to infectious diseases or for patients using high-risk devices. The CDC recommends active monitoring for high-risk exposures because it catches problems early. Institutions using this method saw 37% fewer transmission events during the pandemic. Passive monitoring is self-directed. You check yourself daily and only report if something changes. This works well for low-risk situations, like a minor drug recall or a non-critical device update. Most people use passive monitoring because it’s less intrusive. But here’s the catch: passive monitoring only works if you’re consistent. If you skip a day, you might miss the first sign of trouble. Set a daily alarm. Write it in your calendar. Make it part of your morning routine - like brushing your teeth.Track Symptoms Accurately - Not Just “I Feel Off”
Vague reports like “I don’t feel right” don’t help doctors. You need specifics. Use a simple system:- What - Name the symptom: headache, rash, nausea, shortness of breath.
- When - Time of day it started and how long it lasted.
- How bad - Rate it 0-10. Zero is nothing. Ten is unbearable.
- Triggers - Did it happen after taking the medication? After exercise? After eating?
- Relief - Did anything help? Rest? Painkillers? Water?
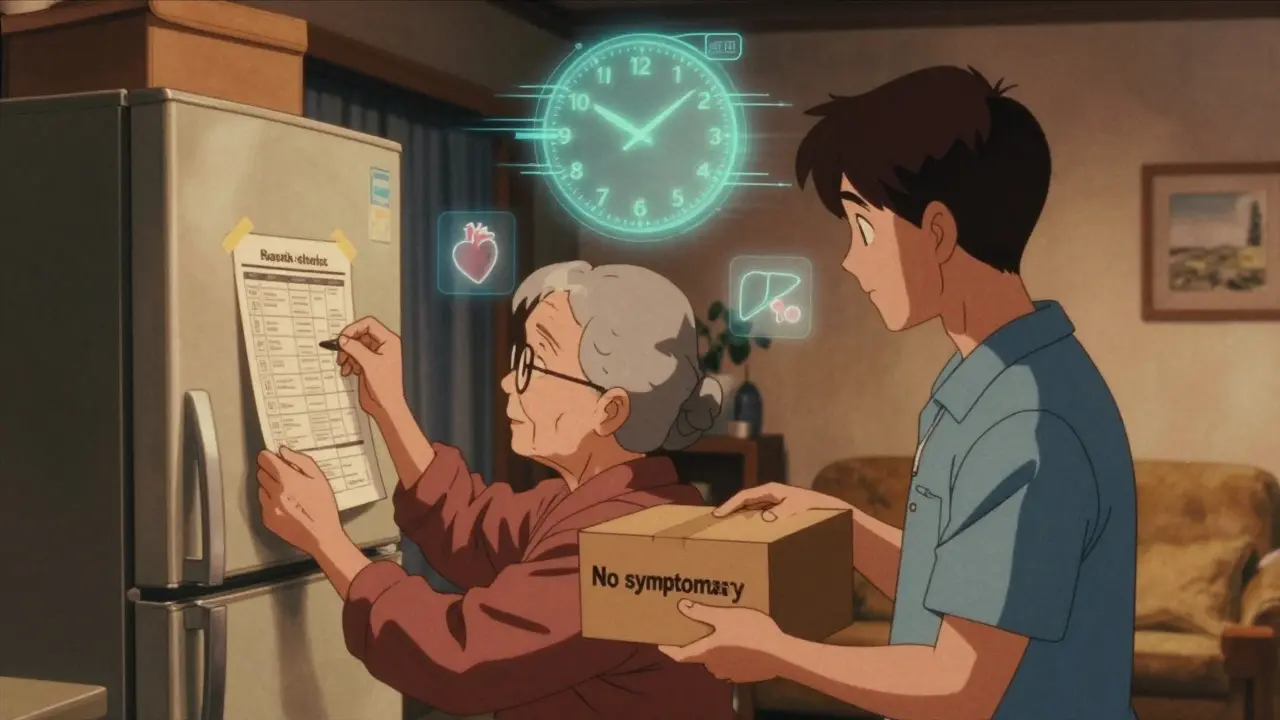
Use the Right Tools - But Watch Out for Pitfalls
There are apps for this. The CDC’s v-safe system, used for COVID-19 vaccine monitoring, sends daily text reminders and asks for symptom ratings. Many hospitals now use EHR-integrated tools from Epic or Cerner. But not all apps are safe. A 2021 HHS report found 67% of symptom-tracking apps didn’t meet HIPAA privacy standards. If you’re using a third-party app, ask: Is my data encrypted? Can the company sell it? Do they share it with insurers? For most people, a paper journal or a basic notes app on your phone is fine - as long as you’re consistent. Write it down. Don’t trust your memory. Even if you feel fine today, write “no symptoms.” That record matters. Older adults or those without smartphones may need help. A 2022 VA study found seniors needed an average of 3.2 training sessions just to use digital tools. If you’re helping someone older, sit with them. Walk through one day of logging symptoms. Make it easy.Know When to Act - Don’t Wait
Monitoring isn’t just about logging data. It’s about knowing when to call for help. The CDC’s guidelines say: if a symptom is new, worsening, or lasts more than 48 hours, contact your provider immediately. Don’t wait for your next appointment. Don’t assume it’s “just a cold.” Use the SBAR method to communicate clearly:- Situation: “I’m calling because I started having chest pain after taking Drug X.”
- Background: “I’ve been on it for 3 weeks. No prior heart issues.”
- Assessment: “The pain is sharp, 6/10, happens after meals.”
- Recommendation: “Can we check my heart enzymes or stop the drug?”
Don’t Fall Into the Alert Fatigue Trap
Too many alerts, too many check-ins, too many notifications - it’s exhausting. A 2022 AMA survey found healthcare workers received an average of 8.2 daily check-ins during peak alerts, but said 4.7 was the maximum they could handle without burning out. If you’re overwhelmed, talk to your provider. Ask: “Can we reduce the frequency?” or “Is this level of monitoring still necessary?” Also, don’t ignore symptoms just because you’ve been fine for days. One false negative can cost you. A 2023 Johns Hopkins study found AI symptom checkers were 22% less accurate for non-English speakers. Human vigilance still matters.Document Everything - For Your Records and Others’ Safety
Keep your symptom log for at least two years. The FDA requires manufacturers to keep monitoring data for that long. But you should too. If you develop a long-term issue linked to the alert, your log becomes evidence. It helps your doctor. It helps researchers. It helps others avoid the same problem. If you’re part of a workplace exposure program, your employer must keep records for 30 years under OSHA rules. Make sure you get a copy of your own log.What If You Miss a Day?
Everyone forgets. Life happens. If you miss one day, don’t panic. Just restart the next day. Don’t try to guess what you felt yesterday - that leads to bad data. Write “missed day - no symptoms recorded.” Honesty matters more than perfection. If you miss multiple days, contact your provider. They may need to adjust your monitoring plan.Why This Matters - Beyond Your Health
Monitoring your symptoms isn’t just about protecting yourself. It’s part of a larger system. Every symptom you report helps regulators spot patterns. It helps manufacturers fix devices. It helps scientists understand risks. During the pandemic, early symptom reports from individuals helped identify rare blood clots linked to certain vaccines - leading to updated guidelines and saved lives. Your vigilance doesn’t just protect you. It protects the whole system.If you’ve received a safety communication, you’re not alone. You’re part of a network of people who care enough to pay attention. That’s powerful. Keep going. Track. Report. Act.
What should I do immediately after receiving a safety communication?
Stop using the product or taking the medication if instructed. Then, review the official alert from the FDA, CDC, or WHO for the exact symptoms to watch for. Write them down. Set up a daily reminder to check for those symptoms. If you’re unsure, call your doctor or pharmacist - don’t guess.
Can I use a smartphone app to track my symptoms?
Yes, but be careful. Apps like v-safe are FDA-approved and HIPAA-compliant. Most third-party apps are not. Check if the app is linked to a hospital, government agency, or reputable health system. Avoid apps that ask for unnecessary permissions or don’t explain how your data is used. If in doubt, use a paper journal or phone notes.
How long should I monitor my symptoms?
It depends on the alert. For drugs, monitor for at least 2-4 weeks after starting or changing dosage. For medical devices, monitor for 3-6 months or as specified in the communication. For infectious disease exposures, follow CDC guidelines - usually 14 days for most viruses. If symptoms appear, continue monitoring until cleared by a doctor.
What if I develop symptoms but the alert didn’t list them?
Report it anyway. Safety communications don’t always list every possible side effect. If you notice something unusual - especially if it’s new, persistent, or worsening - contact your provider. Your report could help update the alert and protect others. The FDA’s MedWatch program accepts voluntary reports from the public.
Do I need to report my symptoms to anyone besides my doctor?
If it’s a drug or medical device, yes. Submit a report to the FDA’s MedWatch program. It’s free, anonymous, and helps improve safety for everyone. You can do it online at fda.gov/medwatch. Your doctor may also report it, but don’t assume they will. Take the extra step.
I’m elderly and find digital tools hard to use. What’s my best option?
Use a printed checklist. Write down symptoms daily with a pen. Keep it by your bed or kitchen table. Ask a family member, caregiver, or pharmacist to help you check it weekly. Many pharmacies offer free symptom tracking sheets. You don’t need tech to stay safe - just consistency and support.
Can symptom monitoring lead to discrimination at work or by insurers?
There’s a real risk. A 2023 Harvard survey found 63% of people feared their symptom data could be used against them - like being denied insurance or fired. Federal laws like GINA and HIPAA offer some protection, but gaps remain. Never share your symptom logs with employers unless required by law. Ask your doctor how data is stored and who can access it. If you feel pressured, contact a patient advocate.



Erika Putri Aldana - 20 December 2025
Ugh, another government pamphlet disguised as self-help. I read this like it was a cereal box. "Monitor symptoms." Yeah right. I got a headache and they want me to rate it 0-10? Like I'm some kinda robot. 🤖
Meanwhile my doctor won't even return my texts unless I'm bleeding. Who the hell is this for? The FDA doesn't even know what day it is.
Adrian Thompson - 20 December 2025
Let me guess - this was written by a CDC contractor who got paid in Starbucks gift cards and vague buzzwords.
"Active monitoring"? That's just surveillance with a wellness veneer. You think they're keeping your data safe? Nah. They're feeding it to Big Pharma's predictive AI so they can jack up prices before you even get sick. HIPAA? More like HIP-NOPE.
And don't get me started on "v-safe." That app's got more backdoors than a 2003 Dodge Charger. I'd rather track my symptoms with a compass and a Ouija board.
Southern NH Pagan Pride - 22 December 2025
Okay but have you considered that these "safety communications" are actually part of a larger psyop? The FDA doesn't issue alerts - they *manufacture* them. Look at the timing. Always right after a stock dip. Always after a new patent expires.
And the "0-10 scale"? That's not medical - that's behavioral conditioning. They want you to quantify your pain so they can normalize it. Soon they'll be requiring you to submit your cortisol levels via QR code.
Also... why does every official site have .gov but the apps are all .com? Coincidence? I think not. 🕵️♀️
Orlando Marquez Jr - 24 December 2025
While the article presents a commendably structured framework for individual symptom surveillance in the context of public health advisories, one must acknowledge the systemic asymmetries in implementation. The burden of documentation is disproportionately placed upon the layperson, while institutional accountability remains opaque.
Moreover, the reliance on digital platforms-despite documented noncompliance with HIPAA-exacerbates health inequities for elderly, low-income, and digitally marginalized populations. A truly ethical system would prioritize accessible, analog, and community-supported monitoring infrastructures over technocratic solutions that privilege convenience over equity.
Jackie Be - 26 December 2025
YESSSSSS this is what we NEED right now!!!
Stop ignoring your body!!! Write it down!!! Even if you feel fine write NO SYMPTOMS!!!
My cousin didn't and now she's in the hospital with liver damage from a drug she thought was "fine"
YOU ARE THE FIRST LINE OF DEFENSE!!!
Get that journal!! Set that alarm!! Be the hero of your own story!!!
And if you're old like me and can't use apps?? GET A PEN AND PAPER!! IT'S NOT HARD!!!
WE GOT THIS!!! 💪🔥
Cameron Hoover - 26 December 2025
This is actually really well put together. I've been tracking my blood pressure meds since the alert came out and using the 0-10 scale. It's weird how much clearer things become when you stop saying "I feel weird" and start saying "headache, 7/10, 3pm, after lunch, eased with water."
Turns out my body's been signaling me for weeks. I just wasn't listening.
Thanks for the reminder that paying attention isn't paranoia - it's self-respect.
Teya Derksen Friesen - 26 December 2025
As a public health nurse in British Columbia, I’ve seen firsthand how structured symptom logging transforms outcomes. One patient, an elderly man with a recalled pacemaker, caught a subtle arrhythmia pattern by writing down his heart rate each morning - before he even felt dizzy.
His log wasn’t fancy. Just a notebook. But it saved his life.
Technology helps, but consistency and human care matter more. Keep it simple. Keep it real. And never underestimate the power of a pen.
Jason Silva - 27 December 2025
Okay but why are we trusting the same people who gave us vaping pods and opioid pills? 😅
I’m logging my symptoms - yeah, for sure. But I’m also screenshotting everything and sending it to my lawyer, my Reddit group, and my cousin who works at the FDA. Just in case.
Also - if you’re using an app? Use Signal to send your logs to yourself. Don’t let Big Tech profit off your sweat and anxiety. 🛡️❤️🩹
And if your doctor says "it’s probably nothing"? Ask for the blood test anyway. I did. Turns out it was something. 🤫