When you're managing epilepsy, even a small change in your medication can mean the difference between staying seizure-free and facing a dangerous breakthrough. For many people, switching from a brand-name antiseizure medication to a generic version seems like a simple cost-saving move. But for those with epilepsy, it’s not that simple. Generic substitution of antiseizure drugs - especially those with a narrow therapeutic index (NTI) - carries real, documented risks that go beyond price tags and pharmacy policies.
Why Antiseizure Medications Are Different
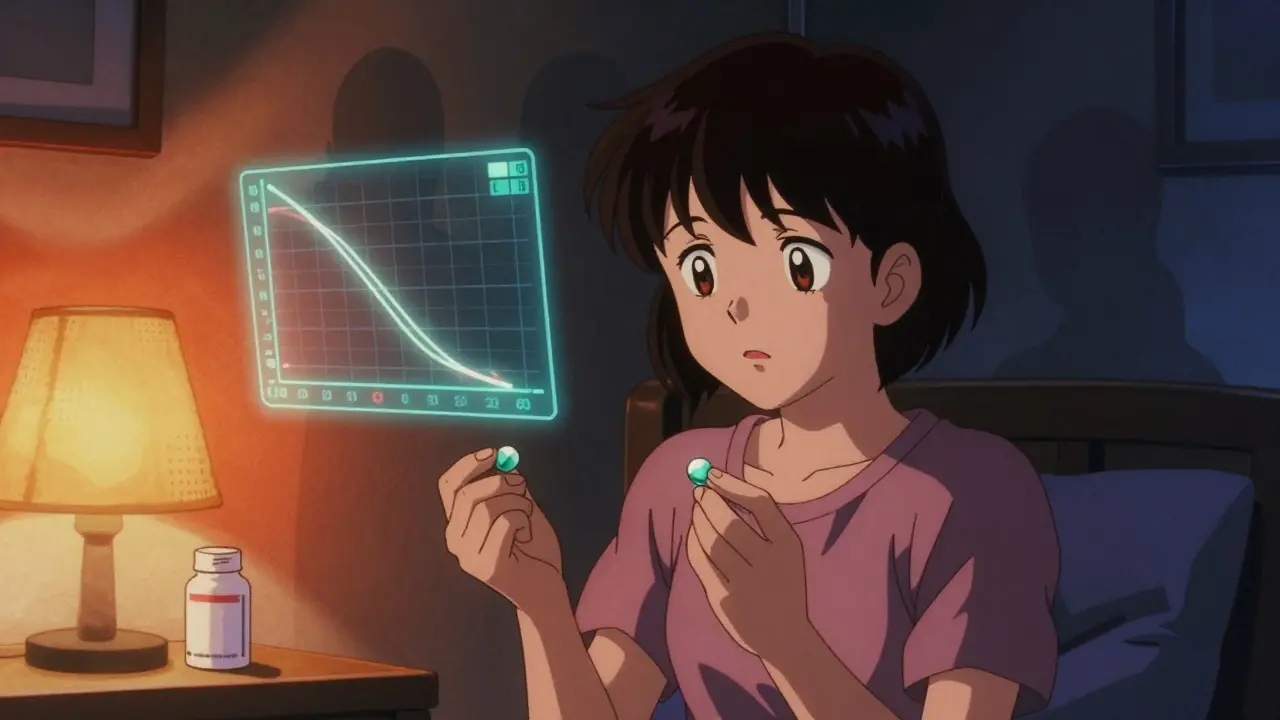
Not all medications are created equal when it comes to generics. Most drugs, like high blood pressure pills or antibiotics, can be safely switched between brand and generic versions without issue. But antiseizure medications (ASMs) are in a different category. Many of them - including lamotrigine, carbamazepine, valproic acid, and phenytoin - have a narrow therapeutic index. That means the gap between an effective dose and a toxic one is very small. A 15% drop in blood levels might cause a seizure. A 15% rise might cause dizziness, confusion, or even liver damage. The FDA says generics must be bioequivalent to the brand-name version. That means the amount of drug absorbed into your bloodstream (measured by AUC and Cmax) must fall within 80-125% of the original. Sounds tight, right? But for NTI drugs, that 45% range is too wide. Two generic versions of the same drug, both approved by the FDA, can still have different absorption rates because of differences in fillers, coatings, or manufacturing processes. These aren’t mistakes - they’re legal under current rules. But for someone with epilepsy, they can be dangerous.What the Data Shows
Real-world evidence tells a different story than regulatory assurances. A 2008 study published in Neurology found that patients who switched from brand-name lamotrigine to a generic version had a 23% increase in doctor visits and an 18% rise in hospitalizations. Another global survey of over 1,200 healthcare providers in 68 countries found that 40% of respondents had seen an increase in seizures linked to generic switches. And it’s not just doctors noticing - patients are speaking up too. On the Epilepsy Foundation’s online forum, one user wrote: "I’d been seizure-free for five years. The pharmacy switched me to generic Lamictal. Two weeks later, I had three seizures. I went back to the brand. They stopped." A Reddit thread from a user named u/ControlledChaos89 described how changing pill colors and shapes caused anxiety so severe it triggered their first seizure in two years. These aren’t rare anecdotes. A 2021 survey by the International League Against Epilepsy found that 68% of patients feared generic substitution, and 42% would pay more out-of-pocket to avoid it. Even more telling: switchback rates. A 2018 study in Epilepsia found that 27% of patients who switched to a generic ASM ended up switching back to the brand name - compared to just 12% for other types of medications. That’s not a fluke. That’s a pattern.Regulatory Gaps and Global Differences
The FDA maintains that its current standards are sufficient. But other countries don’t agree. The European Medicines Agency uses stricter bioequivalence limits - 90-111% - for NTI drugs. That’s a much tighter window. The UK’s MHRA explicitly warns that consistency of supply is critical for ASMs because "the consequence of therapeutic failure might have serious clinical consequences." In places with weaker regulatory oversight, the problem is worse. Some generic versions in low-income countries don’t even meet basic quality standards. The WHO lists carbamazepine, phenobarbital, and valproic acid as essential medicines - meaning they’re vital for global health - yet these are also the ones most frequently linked to substitution problems. And here’s the catch: in the U.S., generics make up about 90% of ASM prescriptions. But in specialized epilepsy centers, where patients have complex cases, doctors often insist on keeping the same brand. Why? Because they’ve seen what happens when the pill changes.Who’s at Highest Risk?
Not everyone who takes antiseizure meds needs to avoid generics. But some groups are far more vulnerable:- People with frequent or uncontrolled seizures
- Those on multiple antiseizure drugs (polytherapy)
- Patients with cognitive impairments or memory issues
- Children and older adults
- People whose seizures are triggered by stress or anxiety
- Anyone on the ketogenic diet - some generic fillers contain hidden carbs that can disrupt metabolic balance
Best Practices for Safe Substitution
If you’re considering or have been switched to a generic ASM, here’s what you need to do:- Ask your neurologist before any switch. Don’t let a pharmacist or insurance company decide this for you. Your neurologist knows your seizure history, your medication response, and your risk profile.
- Request a consistent formulation. If you’ve been stable on a specific brand or generic version, ask your doctor to write "Dispense as written" or "Do not substitute" on the prescription. This legally prevents the pharmacy from switching without your doctor’s approval.
- Check the pill appearance. If your pill changes color, shape, or markings, ask why. Write down the new imprint code and compare it to your previous version. Even small changes can signal a different manufacturer.
- Monitor closely after a switch. Keep a seizure diary for at least 30 days after switching. Note any new side effects - dizziness, nausea, mood changes, fatigue - and report them immediately.
- Know your rights. In many states, pharmacists are required to notify you when a generic substitution occurs. If they don’t, ask. You have the right to know what you’re taking.
What About Cost?
Yes, generics are cheaper. Often 30-80% less. But if switching leads to a seizure, the cost skyrockets. Emergency room visits, ambulance rides, lost wages, missed work, caregiver time - these add up fast. For many families, the long-term cost of a single breakthrough seizure far exceeds the savings from a generic. Some patients worry they can’t afford the brand name. That’s where programs like the Epilepsy Foundation’s Medication Access Program come in. They help over 12,000 patients annually get brand-name ASMs at low or no cost. Don’t assume you can’t afford stability - ask for help.The Future of ASM Treatment
The FDA is considering new guidelines that would tighten bioequivalence standards for NTI drugs to 90-111%, matching Europe’s approach. That’s a step in the right direction. But it’s not enough. The real solution is personalized care. Newer ASMs like cenobamate and fenfluramine have complex pharmacokinetics. They’re not just about blood levels - they’re about how your body metabolizes them over time. For these drugs, consistency isn’t just preferred - it’s essential. The 2024 International Epilepsy Guidelines now recommend individualized risk assessments before any substitution. That means doctors should consider your seizure type, frequency, age, other medications, and mental health - not just your insurance formulary.Final Thoughts
Generic substitution for antiseizure medications isn’t a one-size-fits-all decision. It’s a medical choice - not a pharmacy policy. For many people, switching is safe. For others, it’s a gamble with their safety. If you’re on an antiseizure drug, don’t let cost or convenience override your health. Talk to your neurologist. Know your medication. Track your symptoms. And never assume a generic is the same just because it’s labeled as such. Your brain doesn’t care about the label - it cares about the dose.Can I switch from brand-name antiseizure medication to a generic without risk?
For some people, yes - but not for everyone. Antiseizure medications with a narrow therapeutic index (like lamotrigine, carbamazepine, and valproic acid) are sensitive to small changes in blood levels. Even FDA-approved generics can have different absorption rates due to inactive ingredients or manufacturing differences. If you’ve been stable on a brand-name drug, switching may increase your risk of breakthrough seizures or side effects. Always consult your neurologist before making any change.
Why do some pharmacies switch my medication without telling me?
In most states, pharmacies are allowed to substitute generic versions unless the prescription says "Dispense as written" or "Do not substitute." Insurance plans often push for generics to cut costs. Pharmacists aren’t required to notify you unless state law says otherwise. To prevent unwanted switches, ask your doctor to write "Do not substitute" on your prescription. You can also ask the pharmacy to confirm the brand or generic before filling.
What should I do if I have a seizure after switching to a generic?
Contact your neurologist immediately. Keep a record of when the switch happened, what the pill looked like, and any symptoms you experienced before and after the seizure. Many neurologists will recommend switching back to your previous formulation. Don’t wait to see if it was a one-time event - breakthrough seizures can be dangerous and may indicate that the new version isn’t working for you.
Are all generic antiseizure medications the same?
No. Even two generics of the same drug from different manufacturers can behave differently in your body. Differences in fillers, coatings, and manufacturing processes can affect how quickly the drug is absorbed. One generic might release the medication slowly, while another releases it faster - even if both meet FDA bioequivalence standards. That’s why consistency matters. If you find a generic that works, stick with it. Don’t switch between different generic brands unless your doctor approves.
Can I get help paying for brand-name antiseizure medication?
Yes. Many pharmaceutical companies offer patient assistance programs for brand-name ASMs. The Epilepsy Foundation’s Medication Access Program helps over 12,000 people each year get brand-name drugs at low or no cost. You can also check with nonprofit organizations like NeedyMeds or the Patient Access Network Foundation. Don’t assume you can’t afford stability - help is available.
Why do some neurologists refuse to allow generic substitutions?
Because they’ve seen the consequences. Neurologists who treat complex epilepsy cases often work with patients who have had multiple failed treatments. A single seizure can undo months of progress. Studies show that patients switched to generics have higher rates of hospitalization and emergency visits. For these doctors, consistency isn’t a preference - it’s a safety protocol. They’re not against generics; they’re against unnecessary risk.
Is there a list of antiseizure medications that are high-risk for substitution?
Yes. The most commonly reported high-risk ASMs include lamotrigine, carbamazepine, phenytoin, valproic acid, and phenobarbital. These drugs have narrow therapeutic windows and are on the WHO Essential Medicines List. Newer drugs like cenobamate and fenfluramine also have complex absorption profiles and should be treated with caution. Always ask your doctor whether your specific medication is considered high-risk for substitution.



Liz MENDOZA - 27 December 2025
My cousin just had a breakthrough seizure after her pharmacy switched her lamotrigine without telling her. She’s been stable for 4 years. Now she’s scared to leave the house. This isn’t just about cost-it’s about safety. If your doctor says don’t switch, fight for it.
I’ve seen too many people get sidelined by insurance bots. You deserve to be heard.
Jane Lucas - 29 December 2025
i switched to generic and had a seizure in my sleep. woke up with a bruise on my forehead and no idea how it happened. never again. just sayin.
Kylie Robson - 30 December 2025
From a clinical pharmacokinetics standpoint, the 80–125% AUC bioequivalence window is statistically valid for most drugs, but for NTI agents like phenytoin and carbamazepine, the inter-individual variability in CYP450 metabolism makes even minor shifts clinically significant. The FDA’s one-size-fits-all model ignores CYP2C9 and CYP3A4 polymorphisms that disproportionately affect epileptics.
Also, excipients like lactose or corn starch in generics can alter gastric transit time-especially in patients on ketogenic diets. This isn’t anecdotal; it’s biopharmaceutics.
dean du plessis - 30 December 2025
man i never thought about this till i read this. my buddy in cape town got switched to some generic phenobarbital and started zoning out during work. turned out the batch had different fillers. he lost his job. now he pays out of pocket for the brand. no joke.
weird how a pill can change your whole life
Satyakki Bhattacharjee - 1 January 2026
People are too lazy to take responsibility. If you can’t afford medicine, don’t get sick. The system is broken because we let it be. You want safety? Pay for it. No one owes you a free ride. This isn’t a right-it’s a privilege.
Alex Lopez - 2 January 2026
Oh, so now the FDA is the villain because generics aren’t perfect? Let me guess-next you’ll be blaming Big Pharma for not making every drug 100% identical across 47 manufacturing plants in 12 countries.
Meanwhile, in the real world, 90% of prescriptions are generic because people need access. Yes, there are edge cases. But let’s not turn every epilepsy patient into a special snowflake because a pill changed color. The data shows most switches are fine. The outliers? They get attention. The rest? They’re fine.
Also, "Do not substitute"? That’s a doctor’s job to write, not a patient’s to demand. If your neurologist is worth their salt, they’ll do it.
Caitlin Foster - 3 January 2026
OMG YES. I switched to generic lamotrigine and my brain felt like it was wrapped in wet socks for 3 weeks. I had panic attacks. I cried in the shower. I thought I was losing my mind.
Turns out? The generic had a different binder. My neurologist said "you’re not crazy, you’re pharmacologically violated."
Now I pay $80/month extra because I’d rather pay than die. And if your insurance says no? Tell them to take a hike. Your life is not a spreadsheet.
Elizabeth Alvarez - 3 January 2026
This isn’t about generics. It’s about the FDA being bought by Big Pharma. They let the 80-125% window because the same companies that make brand-name drugs also own the generic factories. It’s all one big cartel.
Did you know the FDA doesn’t inspect most overseas generic plants? Not even once a year. And the fillers? Some are made from ground-up plastic pellets. I’ve seen the documents. They’re hiding it. They want you to have seizures so you’ll go back to the brand-and they’ll raise the price again.
They’re testing us. Like lab rats. And your pharmacy? They’re in on it too. They get kickbacks for switching. Don’t trust anyone. Not the doctor, not the pharmacist, not the government. Only your own seizure diary.
Miriam Piro - 4 January 2026
They’re lying to us. The FDA doesn’t test real-world absorption. They test in healthy volunteers on an empty stomach. But what about people with gastroparesis? With IBS? With ketogenic diets? With anxiety-induced gut spasms? No one cares.
And the color changes? That’s not just filler differences-that’s intentional. They want you to panic. To doubt yourself. To think it’s in your head.
Remember the 2018 study? 27% switched back? That’s not a fluke. That’s a rebellion. And they know it. That’s why they’re pushing new laws to make "dispense as written" harder to get.
They’re eroding our autonomy. Slowly. Quietly. One pill at a time.
And if you think I’m paranoid... ask yourself-why did they change the shape of your pill without telling you?
They’re watching. And they’re waiting.
Chris Garcia - 5 January 2026
There is a profound metaphysical truth here: the body does not recognize corporate logos, nor does it care for shareholder reports. The soul of a medication resides not in its patent, but in its molecular fidelity.
When a man’s brain-already a fragile cathedral of electrochemical fire-must contend with the whims of a tablet’s cornstarch binder, we have moved beyond pharmacology into the realm of existential vulnerability.
Is it not a tragedy that the most sacred act of self-preservation-maintaining neurological stability-is subject to the lowest common denominator of market efficiency?
Generics are not evil. But to treat them as interchangeable in the context of epilepsy is to reduce human consciousness to a line item on a balance sheet.
We must demand not just better regulation, but a moral reawakening: that the body, especially the epileptic body, deserves more than cost-cutting. It deserves dignity.
And dignity, my friends, does not come in a blister pack stamped with a $3 price tag.
Babe Addict - 5 January 2026
Bro, you’re all overreacting. The FDA’s 80–125% range is based on 50 years of data. If you’re having seizures after a switch, you probably missed a dose or were sleep-deprived or ate a burrito.
Also, phenytoin? That stuff’s been around since the 1930s. People took it from dusty bottles in the 70s and lived. Now you’re crying because the pill is blue instead of white?
And don’t get me started on "dispense as written." That’s just a way for neurologists to charge more. Insurance companies are the real villains here, not generics.
Also, I switched to generic carbamazepine and my seizures got better. Maybe your body just needed a change. Maybe you’re addicted to the brand-name placebo effect.
Chris Garcia - 7 January 2026
Interesting. You say the FDA’s standards are based on 50 years of data-but those standards were written before we understood pharmacogenomics. Before we knew that CYP2C9*3 variants affect lamotrigine clearance by 40%. Before we knew that some generics use microcrystalline cellulose from different botanical sources, altering dissolution kinetics.
You blame sleep deprivation? Fine. But why do 68% of patients fear substitution? Why do 42% pay more to avoid it? Why do 27% switch back?
It’s not placebo. It’s physiology. And if your seizures improved after switching, congratulations. But your experience is not the universal truth. It’s one data point in a sea of human vulnerability.
Maybe you’re right. Maybe your body adapted.
But what about the woman who lost her license? The teenager who had a seizure in class? The veteran who can’t work because his brain can’t handle the variability?
Are their lives less important because they don’t fit your anecdote?